Lumoxiti, also known as Moxetumomab pasudotox, is an investigational anti-CD22 recombinant immunotoxin that was made to be used as a treatment for people with relapsed or refractory hairy cell leukemia (HCL) who had been treated with at least two different types of treatment.
The Hairy Cell Leukemia Drugs Development Market a pharmaceutical company based in the United States, following its discovery by the National Cancer Institute.
In April 2018, Medimmune submitted a biologics license application (BLA) to the FDA in the United States. In September 2018, the drug was approved by the FDA as part of its priority review program.
The European Medicines Agency (EMA) has also designated Lumoxiti (Moxetumomab pasudotox) as an orphan drug for the treatment of HCL.
Causes and symptoms of hairy cell leukemia (HCL) are a rare, chronic blood cancer in which the bone marrow produces too many abnormal B cells, preventing the production of healthy blood cells.
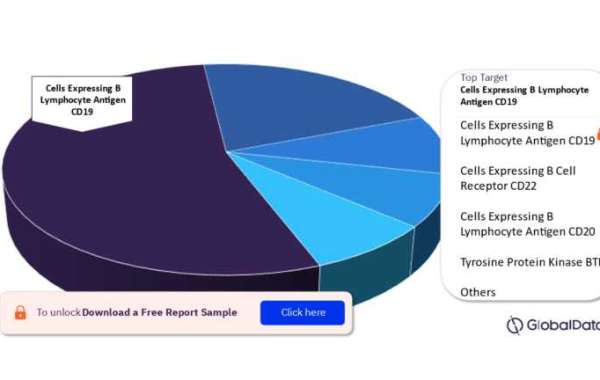
To know about the assumptions considered for the study, Download for Free Sample Report
Under a microscope, HCL patients' abnormal B cells appear hairy. Although the disease's cause is unknown, research suggests that HCL is more likely in people who have been exposed to radiation and certain chemicals used in agriculture and industry.
The disease typically presents with bruising and bleeding, a persistent infection, weight loss, fatigue, weakness, and an uneasy feeling in the abdomen.
HCL is diagnosed in approximately 1,000 Americans annually. The disease has a high relapse rate and very few treatment options.
Mechanism of action of Lumoxiti (Moxetumomab pasudotox) Lumoxiti is an experimental recombinant immunotoxin against CD22. “Research suggests that exposure to radiation and certain agricultural and industrial chemicals increases the likelihood of HCL.” It consists of a binding portion of anti-CD22 antibody fused to a toxin known as PE38, which is a 38kDa fragment of Pseudomonas exotoxin A.
The medication acts as an inhibitor of the CD22 antigen, binding to CD22 to release the toxins into the cell. The cancer cells die because the toxin prevents them from translating proteins.
Cancer patients receive intravenously administered recombinant immunotoxin to ensure that the medication reaches every cell.
Lumoxiti clinical trials The data from the Phase III clinical study "1053" were the foundation for the FDA's approval of Lumoxiti. The open-label, multi-center, single-arm study included 80 patients with relapsed or refractory HCL from 34 countries across 14 countries. Durable complete response was the trial's primary endpoint.
With a 30% durable CR rate in patients receiving Lumoxiti (Moxetumomab pasudotox), the trial met the primary endpoint. Additionally, the drug had a CR rate of 41% and an objective response rate of 75%.
73% of patients had a long-lasting response, and 82% had a negative rate of minimal residual disease (MRD). In addition, 80% of patients experienced hematologic remission.
The median time to reach hematologic remission for the patients was 1.1 months.
During the clinical trial, the most common treatment-related side effects that were observed were nausea, headaches, peripheral oedema, and pyrexia.
Commentary on Medimmune's marketing strategy Medimmune is an AstraZeneca-owned subsidiary with its headquarters in Gaithersburg, Maryland.
Oncology, inflammation, autoimmune diseases, and cardiovascular diseases are the company's primary therapeutic areas. It is working on more than 120 biologics for a variety of conditions, such as cancer, asthma, lupus, and chronic obstructive pulmonary disease (COPD).