Non-Alcoholic Steatohepatitis (NASH) Clinicial Trail analysis is a progressive liver disease characterized by liver inflammation and damage due to fat accumulation, unrelated to alcohol consumption. NASH is part of the non-alcoholic fatty liver disease (NAFLD) spectrum and has become a significant health concern globally due to its association with obesity, type 2 diabetes, and metabolic syndrome.
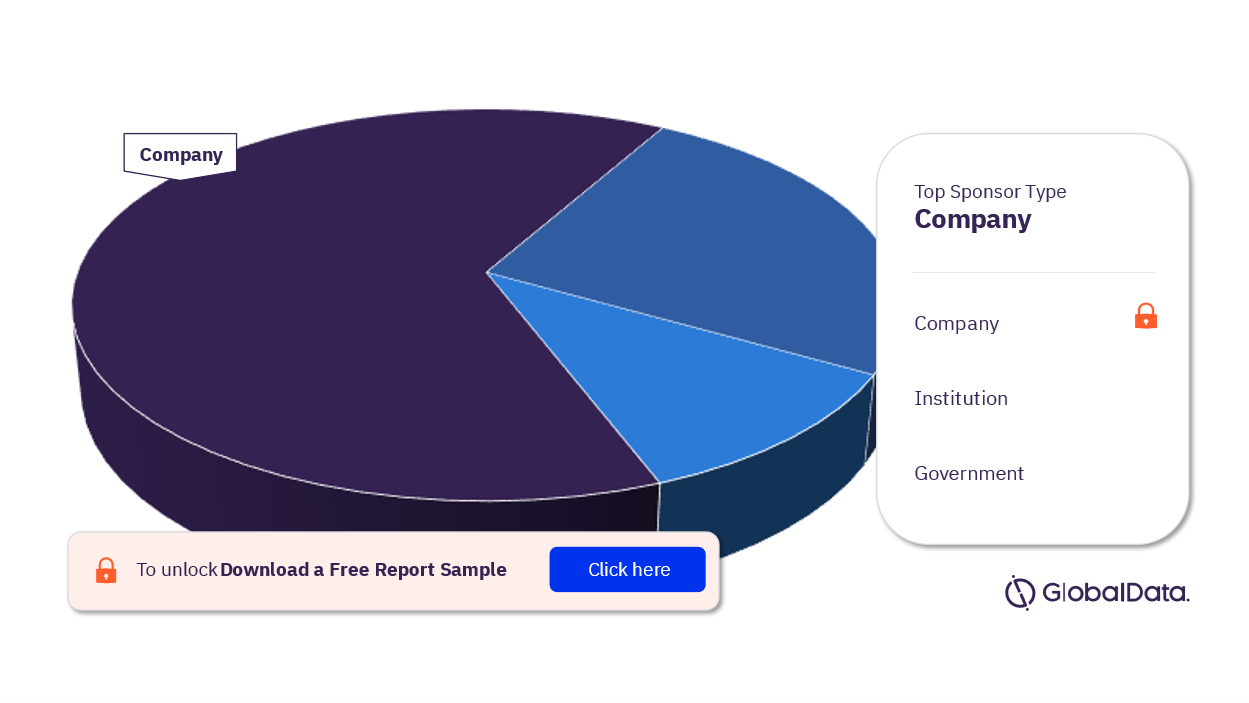
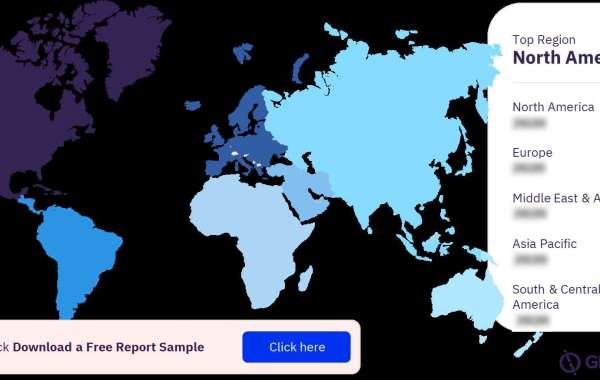
For more sponsor type insights into the NASH clinical trials market, download a free report sample
Key Drivers of NASH Clinical Trials
Rising Prevalence of NASH and Related Conditions: With the rise in obesity, diabetes, and metabolic syndrome cases globally, NASH has become more prevalent. This increase drives the need for effective treatments, spurring interest in clinical trials focused on NASH.
Urgent Need for FDA-Approved Treatments: Currently, there are no approved pharmacological treatments specifically for NASH, creating a substantial unmet need. This lack of therapeutic options motivates pharmaceutical companies to invest in clinical trials aimed at developing targeted therapies.
Advancements in Biomarkers and Diagnostic Tools: The development of new biomarkers and diagnostic tools enables researchers to better identify NASH in its early stages and track treatment responses more effectively. These advancements facilitate the design and execution of more precise clinical trials.
Increased Investment from Pharmaceutical Companies: Given the high disease burden and the lack of approved therapies, pharmaceutical companies have increased investment in NASH research. This is evident in the rising number of clinical trials and partnerships between industry and academia.
Support from Health Authorities: Regulatory agencies like the FDA and EMA have shown support for NASH research by providing guidance on trial design, endpoints, and surrogate markers for clinical trials. This support streamlines the development pathway, encouraging more trials.
Key Phases and Approaches in NASH Clinical Trials
Preclinical Trials: Preclinical research for NASH includes animal studies and in-vitro experiments to understand disease mechanisms and identify promising drug candidates. Researchers often investigate pathways involved in liver fat metabolism, inflammation, and fibrosis to develop targeted therapies.
Phase I Trials: Phase I trials primarily focus on evaluating the safety and tolerability of potential NASH treatments. These trials are crucial for identifying any adverse effects and determining safe dosage ranges for further studies.
Phase II Trials: At this stage, researchers focus on the efficacy of the drug in reducing liver fat and fibrosis. Surrogate endpoints like reductions in liver enzymes and liver fat are commonly used, alongside more invasive assessments, such as liver biopsies, to confirm efficacy.
Phase III Trials: Phase III trials are the final phase before a drug can be submitted for regulatory approval. They involve larger patient populations to confirm efficacy and assess the drug’s safety profile comprehensively. Drugs that demonstrate substantial benefits in Phase III trials may receive FDA Fast Track designation, expediting their path to approval.
Combination Therapy Trials: Given the multifactorial nature of NASH, researchers are exploring combination therapies that target multiple aspects of the disease, such as inflammation, liver fat, and fibrosis. Combination therapies have shown promise in early-stage trials and may offer a comprehensive approach to managing NASH.